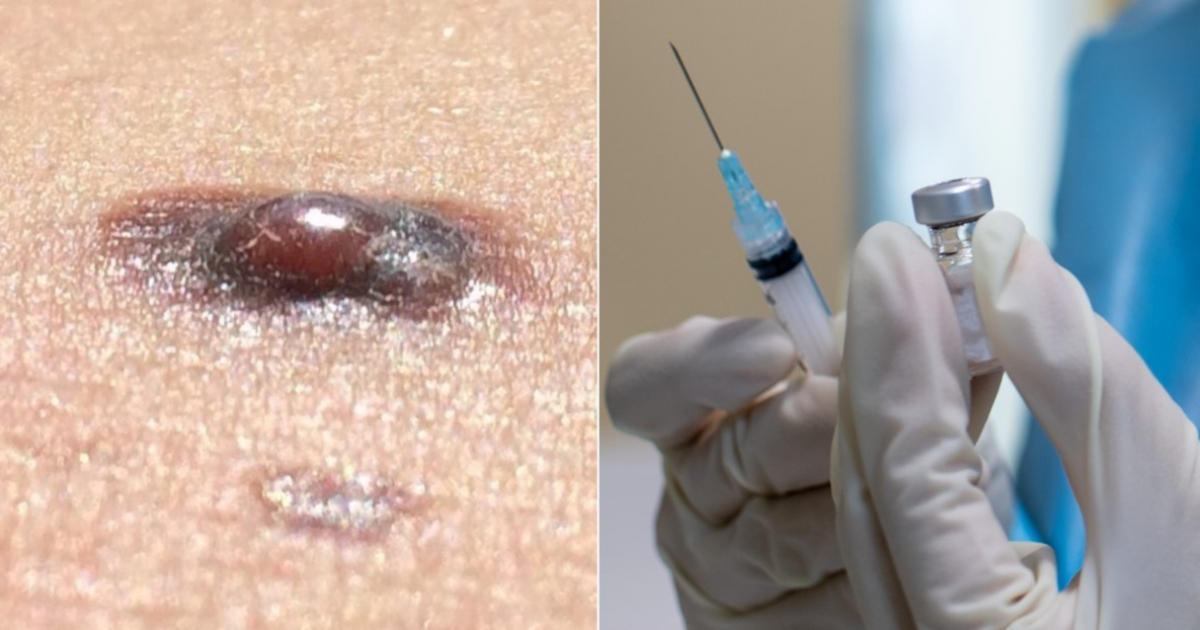
Stéphane Bancel, CEO of the pharmaceutical companyModern, said this Thursday that askin cancer vaccine could obtain regulatory approvals and be launched in some countries by 2025, after presenting the results of preliminary trials (phase 2b) of its therapy against that type of disease.
According to mid-stage trial data published byModernandMerck & Co., when the use of this vaccine is combined with the drug Keytruda, a successful immunotherapy ofMerck, the possibility of death of patients is reduced by 49%.
The experimental messenger RNA vaccine (the same method used to rapidly developcoronavirus vaccines) also reduced the risk of melanoma spreading to other parts of the body by 62%.
In the observations, the specialists noted that the The most common side effects of the vaccine were fatigue, pain at the injection site and chills, according to the experiment data.
In aprevious essay, the vaccine and Keytruda had reduced the risk of death or relapse by44% in patients with melanoma andreduced the risk of cancer spreading through the body by 65%.
"The durability of the responses is really strong, essentially they are rock solid during this time," said Moderna president,Stephen Hoge. "This is a pretty significant improvement, a pretty dramatic improvement over the standard of care with Keytruda alone," the news agency quotedReuters.
Likewise, the executive director of the pharmaceutical company Moderna said on the television program “Squawk Box" ofCNBC, who is eager to see four- and five-year data on the vaccine because “the melanoma curve is flattening, people are no longer dying” after going through treatment.
New results suggest cancer vaccine used with immunotherapy continues to provide significant health benefits. Their design to train the immune system to recognize and attack specific mutations in cancer cells is important because it gives hope to melanoma patients after they remain on treatment for a longer period of time.
ThePhase three of these trials began in July and the two drugmakers are studying the combination as a treatment for late-stage melanoma, reportsCNBC in your report.
For now,Moderna is building a facility in Massachusetts to produce the vaccine on a commercial scale., which he hopes to finish next year. They have also begun a confirmatory trial to treat non-small cell lung cancer that is already enrolling patients.
What do you think?
COMMENTFiled in: