Related videos:
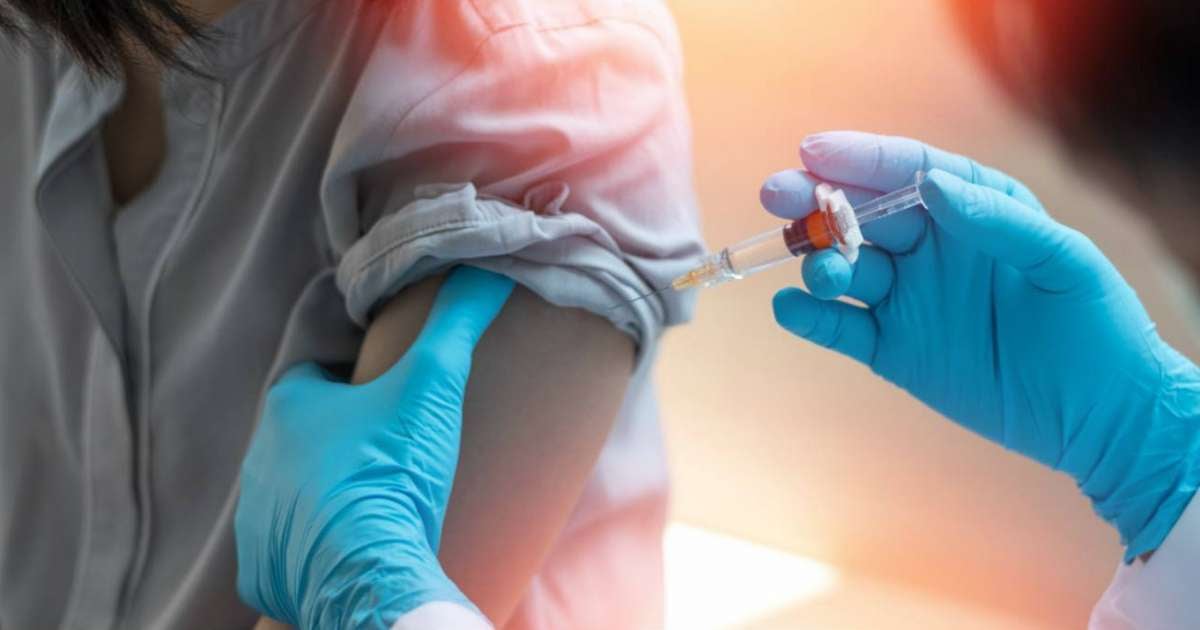
U.S. regulators have approved new doses against COVID-19 but imposed restrictions that complicate access for millions of people and withdrew one of the vaccines designated for young children.
The information was reported by Associated Press (AP), which detailed that the updated doses of Pfizer, Moderna, and Novavax have been approved for older adults, while for minors and young adults, only those with at least one risk condition, such as asthma or obesity, will be able to access them.
This creates new barriers for those who wish to get vaccinated but cannot provide proof of illness.
In parallel, the Pfizer vaccine has been excluded for all children under the age of five, as the FDA revoked the emergency authorization that allowed its use in that age group.
The available alternative will be the Spikevax vaccine from Moderna, approved for children from six months old, but only limited to those with serious health issues.
The Novavax booster is restricted to individuals over 12 years old, under the same risk criteria.
The agency explained that the measure is being adopted following a review of data regarding the evolution of the virus and in response to questions about the necessity of immunizing the entire population every year, as had been done until now.
The new policy breaks with the previous strategy, which recommended annual doses for all Americans from six months of age.
Several medical associations, including the American Academy of Pediatrics, have criticized the decision, warning that it restricts access for families seeking to protect their children.
The changes align with a climate of instability in the public health system. Just this week, the director of the Centers for Disease Control and Prevention (CDC), Susan Monarez, was dismissed by the White House after less than a month in office, reported AP.
A spokesperson claimed that she was not "aligned" with President Donald Trump's agenda, while her lawyers argue that she was removed for defending science in the face of political pressures.
Monarez's departure was accompanied by the resignation of at least four high-ranking officials from the CDC, including those in charge of immunization and infectious diseases.
In farewell letters, some denounced the censorship of scientific communication, budget cuts, and the handing over of key responsibilities to individuals with a history of skepticism toward vaccines.
Public health experts described the situation as a "decapitation" of the CDC and warned that the loss of experienced scientific personnel undermines the country's preparedness for health emergencies.
The new guidelines on vaccines, along with the crisis in leadership at the main health agency, confirm a scenario of uncertainty just as the season for a resurgence of respiratory viruses begins and with tens of thousands of COVID-related deaths recorded in the last year in the United States.
The new vaccination policy in the United States, which excludes individuals without risk conditions and eliminates the use of Pfizer for children under five, is being implemented at a time when the WHO warns about the global increase in cases and the emergence of a new, more transmissible subvariant.
Although with lower lethality, experts warn that the relaxation of health measures and social fatigue are creating opportunities for spikes that could once again overwhelm health systems.
Meanwhile, in Cuba, health authorities have decided to resume the scheme of vaccination with a new booster dose. The campaign includes the administration of national vaccines for those over 50 years old, pregnant women, and immunocompromised individuals, following the detection of a slight increase in infections in several provinces of the country.
This reactivation contrasts with the restrictive approach adopted by the United States, where experts warn that overall immune coverage is being jeopardized by decisions driven more by politics than by scientific evidence.
Frequently Asked Questions about the New Vaccination Restrictions in the U.S.
Why has the United States restricted access to new doses of COVID-19 vaccines?
The restrictions are applied to prioritize individuals with risk conditions, such as asthma or obesity, and to avoid annual general vaccination of the entire population. These measures are based on a review of data regarding the evolution of the virus and concerns about the necessity of immunizing all citizens each year.
What COVID-19 vaccine is available for children under five in the U.S.?
The only vaccine available for children under five years old is Moderna's Spikevax. The FDA has revoked the emergency authorization for the Pfizer vaccine in this age group, limiting options to the Moderna vaccine, which is only administered to those with serious health issues.
How does the dismissal of the CDC director affect vaccination policy in the U.S.?
The dismissal of Susan Monarez and the resignation of other senior officials at the CDC have created a climate of instability in the leadership of this key public health agency. This situation could weaken the country's preparedness for health emergencies and impact the implementation of vaccination policies.
Filed under: